The Clinical Research Network (CRN) is working to improve diverse participation in research by bringing community-engaged staffing services to high priority, under-resourced trials in service to our researcher and patient communities. Our team will provide updates about how we’re engaging with our communities to improve awareness, understanding, and trust in research through outreach, education and building authentic community partnerships.
September 2024
New Clinical Research Coordinator III

On behalf of the Clinical Research Network (CRN) and Center for Clinical Research Advancement (CCRA), we are thrilled to introduce David (Dave) Krause, the newest member of our team.
Dave will serve as a Clinical Research Coordinator III and be responsible for the study coordination of several clinical trials currently directed by the CRN. He brings a wealth of experience (10 years) in research and clinical trial conduct from both the local site and sponsor side of the industry.
Dave most recently worked at University of Massachusetts Medical School in Worcester as a Clinical Research Coordinator successfully conducting clinical trials on the emergency use authorization of the Moderna and Pfizer-BioN Tech Covid-19 vaccines.
Prior to Massachusetts Medical School, David served at Takeda pharmaceuticals as an Industry Research Assistant in the Global Medical Department, specifically, working on Phase I, II, and III Cancer/Oncology trials. Before joining Takeda, David served as a Clinical Research Associate at Centogene where he was a part of a team conducting clinical trials in Parkinson’s Disease, travelling to Rostock, Germany and North Africa.
David earned his Master’s in International Public Health in 2010 from the University of Uppsala in Sweden’s, Department of Maternal and Child Health, contributing to the publication of the study, “A Qualitative study of A Perspectives among the Bolgatanga municipality People of Ghana Perceptions, Attitudes, Experiences and Opinions of Tuberculosis Associated: Int. J. Environ. Res. Public Health 2022, 19(22), 14998; Available online https://doi.org/10.3390/ijerph192214998. After grad school David also conducted research on School Food Programs at public schools in Lynn and Lawrence, Massachusetts for Project Bread at Harvard Chan School of Public Health. Please join us in welcoming Dave to BMC!
August 2024
Community Engagement Updates

Last month, the Clinical Research Network attended the 7th annual Healing Power Event: Celebrating Cancer Survivorship.
Hosted by the Dana-Farber/Harvard Cancer Center, the goal of this event was to celebrate and provide cancer survivors, caregivers, and the general public with knowledge on integrative and complementary therapies. In addition to learning about these techniques, we will provide opportunities for attendees to experience them. The goal was for individuals to experience the importance, benefits, and sampling of integrative and complementary therapies (such as massage, reiki). The event promoted healthy living, good nutrition, and physical fitness, as well as offered a variety of demonstrations, samples, literature, screenings, and speakers.
Dr. Ilori, a nephrologist with BMC/BUMC, was invited to this event to provide urine screenings and education about chronic kidney disease. With Dr. Ilori, the CRN discussed APOL1-mediated kidney disease (AMKD) risks with community members and provided them with information and next steps to understand their kidney health.
We also had the opportunity to discuss kidney disease research options with interested community members. Dr. Ilori is the Co-Investigator on the AMPLITUDE research study, which is aimed at developing a treatment for AMKD.
To learn more about APOL1-Mediated Kidney Disease, please visit: www.kidneyfund.org/apol1aware
To learn more about the AMPLITUDE study, please contact: Titilayo.ilori@bmc.org, Afolarin.amodu@bmc.org
July 2024
Portfolio Updates
The CRN’s goal is to improve diverse participation in research by bringing experienced, community-engaged staffing services to high priority, under-resourced trials in service to our researcher and patient communities. In FY2024, the CRN is nearing our target goal of achieving an average of 70% of enrollment goals across all managed trials. We are currently at 67.5% of enrollment goals for all trials. CRN managed trials have enrolled a total of 97 participants (39 participants in FY24).
Demographics across CRN Trials: 97 total patients enrolled
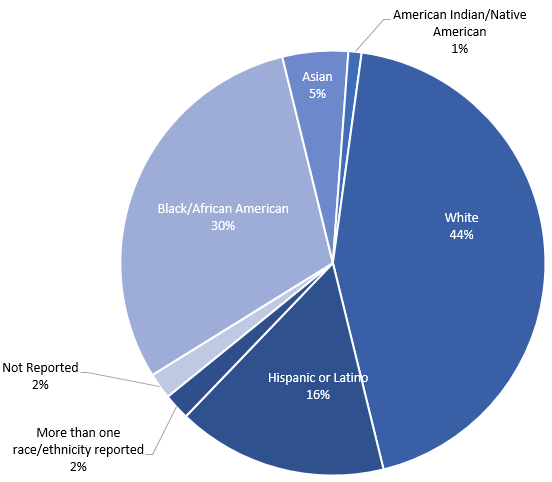
Community Engagement Updates
The CRN has had a busy summer! Be sure to keep up with us on our calendar of community events.
We are proud to have been able to support Boston’s Haitian-American Unity Parade on May 19th. Together with our BMC teams, we celebrated Haiti's independence and helped empower our neighbors by providing resources that support all aspects of health. BMC is committed to being a partner with the Haitian community and fulfilling our mission of empowering all patients to thrive. Thank you to all the organizers for an amazing celebration!

June 2024
Community Engagement Updates
The Clinical Research Network spent a great Sunday afternoon at the Dorchester Open Streets event on May 5th, 2024. The Open Streets events are an opportunity for community members to experience streets as public spaces. Dorchester Avenue was closed to traffic and open to the community from Fields Corner to Ashmont. The street was lined with community organizations, vendors, and activities for families for the 1-mile stretch.
The CRN was joined by the MA-CEAL team and our close partners at We Got Us. We handed out hygiene kits (made possible by our partner, Hope & Comfort!), health information, research education, and information about how to find research studies at BMC/BUMC.
The CRN has a packed schedule for community events this summer – be sure to check out our calendar here to stay up to date on where to find us!
May 2024
Community Engagement Updates
APOL1 Awareness Day
April 30th 2024 – Be APOL1 Aware This AMKD Awareness Day
Are you at an increased risk for developing kidney disease or kidney failure? Your genes could provide the answer.
People with two mutations of the APOL1 gene (apolipoprotein L1) are not guaranteed to develop kidney disease or failure, but there is a 1 in 5 chance they will. The Clinical Research Network is supporting the American Kidney Fund in its efforts to raise awareness about APOL1-mediated kidney disease (AMKD) with the launch of the first annual AMKD Awareness Day on April 30, 2024.
Everyone has the APOL1 gene, but people of west and central African ancestry, including people who are Black, African American, Afro-Caribbean and Latina/Latino, are more likely to have mutations of the gene. In fact, it is estimated that 13% of Black Americans have two of the genetic variations that put them at greater risk of developing kidney disease or failure. Find out more about AMKD by visiting KidneyFund.org/APOL1aware.

The CRN is working with Drs. Amodu and Ilori on an AMKD study sponsored by Vertex called AMPLITUDE. The Amplitude study is exploring an investigational drug called VX-147, to determine if people who get the investigational drug have better outcomes than those who take the placebo.
While there are treatments available to help with the symptoms of APOL1-mediated kidney disease, there are currently no approved treatments that address the underlying cause. As APOL1-mediated kidney disease is genetic, it can affect multiple people in the same family and be passed down to younger generations. This is why clinical research is so important: to try to find a potential treatment that addresses the underlying cause and potentially help create better treatments for those impacted by the disease.
On April 30, join the Clinical Research Network and be APOL1 aware!
If you are interested in learning more about the study, please contact:
Nike Asupoto, Clinical Research Coordinator II: olanike.asupoto@bmc.org
Black Family Wellness Event with BMC & The Links
Boston Medical Center teams showed up strong at the Black Family Wellness Event at the Twelfth Baptist Church in Roxbury on Saturday March 23rd. Hosted by the Links, the event invited community organizations together to share health and wellness resources to our families and community members.
Despite the rain, hundreds of community members showed up to enjoy the day. Dozens of BMC teams provided health and wellness information – Dr. Nyia Noel gave a talk on fibroids and Dr. Julien Dedier discussed kidney disease and diabetes. The CRN provided research education and chronic kidney disease educational materials, including information about APOL1-mediated kidney disease. We also handed out over 80 hygiene kits, made possible by our partner Hope & Comfort!
We were so proud to show up with our fellow BMC teams, like BMC Community Engagement & External Affairs, BU CTSI Community Engagement, MA-CEAL, the Health Equity Accelerator, and dozens of others. Looking forward to the next event!
April 2024
CRN Resource Night: Discussing PTSD Symptoms, Treatments, Resources and Research
At the end of February, the Clinical Research Network (CRN) hosted a resource night with our partner Union Capital Boston (UCB) to have an open and honest conversation about trauma and mental wellness to reduce stigma and create awareness. Our panel of experts included Dr. Karen Abdool, Dr. Ann Rasmusson, Dr. Luke Stalcup, the BMC’s RESTORE Center, and BMC’s MA-CEAL. We were joined by over 150 community members for an engaging discussion about support, treatment, and research opportunities available through Boston Medical Center for individuals impacted by post-traumatic stress disorder (PTSD).
Speaker Spotlight

Dr. Abdool is a psychiatric specialist and mental health provider at Beryllium Psychiatric that specializes in trauma-informed care. We connected with Dr. Abdool through Pure Spark, an organization that connects mental wellness resources with the people who need them. Pure Spark has a directory of Black wellness providers across Massachusetts. Dr. Abdool discussed trauma a physical illness, the impact of trauma in our families and communities, and trauma in African American and other impacted communities.
Contact: kabdool@berylliumpsych.com


Dr. Rasmusson is the Principal Investigator (PI) on an NIMH-supported research study aimed at improving trauma-focused PTSD treatments. She is an expert in treating PTSD and has been doing research for 30 years. Dr. Stalcup is a researcher, a Veteran of the US Army, and a Co-Investigator (Co-I) on the NIMH Trial with Dr. Rasmusson. Dr. Rasmusson discussed the PTSD Allo Study, a clinical trial that is investigating whether a natural anti-stress hormone called “Allo” (allopregnanolone) affects processes involved in recovery from PTSD. Results from this study may help with development of treatments for PTSD. The investigators answered questions from community members about PTSD and PTSD research at BMC. To learn more, contact the team below.
Contact: PTSDAllo@bu.edu
Website: https://redcap.link/rcvq08qb
RESTORE Center
The REcovery from Stress and Trauma through Outpatient care, Research, and Education (RESTORE) Center provides evidence-based mindfulness and cognitive behavioral treatments for posttraumatic stress disorder (PTSD) and other programs focused on resisting oppression-based stress. Joselyn Gil and Zoey Goldberg discussed resources available through the center, including the TAP Study. The TAPS (Treatment for Antepartum PTSD Study) is trying to determine if a brief talk therapy for PTSD will be helpful to pregnant women with symptoms of PTSD. To learn more, contact the team below.
Contact: restore@bmc.org; tapsbmc@gmail.com
Website: www.bmc.org/restore; www.bumc.bu.edu/prismm/research/tap-study/
MA-CEAL
The Massachusetts Community Engagement Alliance (MA-CEAL) Hope White, the Director of Community Engagement, hosted a discussion on the connection between social determinants of health and mental health, and how to connect with resources in your community. To learn more, contact the team below.
Contact: ma-ceal@bmc.org
Website: www.bmc.org/ma-ceal
March 2024
CRN Research Portfolio Update
| Study Name | Goal | PI | Department | Sponsor | Status |
COVID-19 Pediatric Oral Treatment Study | Investigate treatment for COVID-19 in high-risk pediatric patients | Katherine Gergen Barnett | Family Medicine | Pfizer | Active |
Amplitude | Investigate treatment for APOL1-mediated kidney disease (AMKD) | Afolarin Amodu | Nephrology | Vertex | Active |
PTSD Study | Investigate hormone impact on brain processing to develop treatment for PTSD | Ann M. Rasmusson | Psychiatry | NIMH | Active |
RECOVER - VITAL | Investigate treatment for Long COVID (PASC) | Nabila Azad | General Internal Medicine | NIH | Active |
RECOVER - NEURO | Investigate treatment for cognitive symptoms of Long COVID (PASC) | Brigid Dwyer | Neurology | NIH | Start Up |
ESSENCE Trial | Investigate treatment to slow or reduce NASH (non-alcoholic fatty liver disease) | Arpan Mohanty | Gastroenterology | Novo Nordisk | Active |
Community Engagement Update
Liver Health Conversation with Union Capital Boston

On Tuesday February 13th, the Clinical Research Network hosted a conversation at Union Capital Boston’s Partner Night about liver health and research at Boston Medical Center. Our speaker, Dr. Arpan Mohanty, MD, MSc, is an Assistant Professor of Medicine at BUMC and a Hepatologist with BMC. Dr. Mohanty’s focus is on the diagnosis and management of liver diseases.

She is also a clinical researcher working investigating fatty liver disease and cirrhosis, and is the PI on several clinical trials at BMC. The CRN is supporting Dr. Mohanty with the Essence trial, which is investigating a treatment for MASH (Metabolic dysfunction-Associated Steatotic Liver Disease), a progressed form of fatty liver disease. We teamed up with Dr. Mohanty to host an engaging conversation with over 75 community members about their liver health, and how to identify and manage liver disease.
Dr. Mohanty shared health resources, discussed the differences between health research and health care, and how clinical trials play a role in the healthcare system. After discussing the Essence trial, Dr. Mohanty was able to field questions from community members about the trial, about research, and how to find resources to learn more about your liver health.
Upcoming Events:
- Saturday March 23rd @ 10:00 – 2:00 PM
- Black Family Wellness Expo 2024
- Twelfth Baptist Church, Roxbury MA
View our community events calendar on our website for the most updated schedule of events.
January/February 2024
Institutional Research SOPs in 2024 - Training now required
Institutional Standard Operating Procedures (SOPs) for human subjects research are available and have been effective as of January 1st, 2023. These SOPs guide members of the research community at BMC and BU Medical Campus and cover guidance on how to complete some of the daily activities of a research team and also address overarching structural activities. The SOPs are posted within INSPIR II, the electronic IRB application system. To access the SOPs, you will need an INSPIR II account. Once logged in, click the (?) Help Icon in the upper-right hand corner of your INSPIR home page. This will open a separate window that includes links to the SOPs.
Training on the SOPs is now required for 2024 for any individual working on clinical research studies that target BMC patients, use BMC patient data, or utilize BMC facilities and/or services and recommended for anyone working with human subjects or data. See Scope and Definitions below for more detail on who is required to take this training or visit the OHRA landing page on SOPs.
Key dates for SOP training in 2024
- January 1, 2024: all new studies targeting BMC patients, utilizing BMC facilities and/or services, or using BMC patient data will require completion of training prior to IRB approval
- January 1, 2024: new BMC employees working on studies within scope are required to complete training within 90-days of employment start date
- December 31, 2024: existing clinical research investigators and staff are encouraged to complete
Scope and Definitions
The required training courses are available within CITI currently. To access, log into CITI using your Boston University Medical Campus/Boston Medical Center-affiliated account. After choosing ‘View Courses’ for Boston University Medical Campus/Boston Medical Center, navigate to the bottom of the page under the ‘Learner Tools’ heading and choose ‘Add a Course’. SOP Training is listed under Question 10.
There are two tiers of SOP training, Abbreviated and Fundamental. Within the CITI training, there will be a course for each tier level. Researchers should choose which training course to complete based on the tier that applies to their research, as described below. The two tiers are based on the study category that individuals are working on. If you are unsure what categories apply to your research, the initial IRB outcome letter for each study will list the category or categories.
- Abbreviated Training Level: Researchers who only work on studies within Exempt Categories 4, 9, 10 or Expedited Non-Exempt Category 5 will need to complete training on a smaller subset of SOPs. i.e., Abbreviated Training is required if your research only involves use of data or biospecimen collection, with no human subject interaction.
- Fundamental Training Level: Researchers working on any other type of study other than listed above will need to complete training on the full set of SOPs. i.e., Fundamental Training is required if you work on any studies that involve interaction with human subjects.
- For those working on a mix of studies, if at least one study involves interaction with human subjects, Fundamental Training is required.
If there are questions about which level of training to complete, contact the BMC Clinical Research Network (CRN) for guidance. Individual research teams, departments, or other groups wishing to receive customized-SOP training should contact the CRRO for information.
BMC patients, utilizing BMC facilities and/or services, or using BMC patient data are defined as:
- BMC patient: any individual with a clinical encounter generating a BMC-specific medical record
- BMC patient data: patient data that is derived from BMC medical records and/or systems
- BMC facilities: clinical or non-clinical space owned or operated by BMC
- BMC services: a unit or group operated or managed primarily by BMC staff
CRN Seminars and Workshops
As part of our commitment to improving research infrastructure and sharing what we have learned about inclusive research practices, the CRN partners with our colleagues and the research community to disseminate information we hope will be useful.
Recent workshops and seminars
November 9, 2023: I have SOPs and know how to use ‘em!
Background, Required Training and Practical Application of BMC & BU Clinical Research SOPs.
Presenters:
- Mary-Tara Roth RN, MSN, MPH: Director of Clinical Research Resources Office
- Ryan Schroeder, Director – Clinical Research Network
- Duncan Schulte, Regulatory Project Manager, Clinical Research Network
January 10, 2024: Language Diversity in Research – Resources & Tips
Resources and best practices to overcome known language barriers in research at BMC.
Presenters:
- Ryan Schroeder, Director – Clinical Research Network
- Johanna Chesley, Sr. Director – Center for Clinical Research Advancement
- Duncan Schulte, Regulatory Project Manager, Clinical Research Network
- Quinneil Simmons, Program Manager, Clinical Research Network
Upcoming workshops and seminars
March 2024: Clinical Trial Budgeting and Financial Management – Demystifying the Costing and Negotiation Process to Optimize Study Budgets and Manage Awards
A deep dive into managing the costing and budget negotiation process in trials and clarify roles and resources.
Presenters:
- Ryan Schroeder, Director – Clinical Research Network
- Michael Porreca, Sr. Manager, Clinical Trial Office
- Quinneil Simmons, Program Manager, Clinical Research Network
Community Engagement Updates
Long COVID Ambassador Training
• Interested in becoming a health ambassador in your community? The MA-CEAL and CRN teams are working together to host trainings for community members to become health ambassadors in their community. Ambassadors will learn about Long COVID, its symptoms, and treatments available. Compensation is provided. If you have community outreach experience or are interested in becoming an ambassador and attending a training, please fill out the form here: https://redcap.link/LCAT
Upcoming Community Events
- February 12th & 13th @ 9:00-1:00PM
- Long COVID Ambassador Training, Fill out the interest form
View our community events calendar on our website for the most updated schedule of events.
December 2023
Community Engagement Updates
On December 7th, the Clinical Research Network attended the Union Capital Boston’s (UCB) Resource Night hosted by BU CTSI’s Community Engagement Program. This event was focused on providing community members with a foundation in clinical research processes and history, and share resources at BMC/BUMC in research and healthcare. Ridiane Denis, RN, MD, from the GCRU (General Clinical Research Unit) and Kareem King from BU CTSI provided
With Dr. Jai Marathe, the CRN provided information on Long COVID, and discussed the RECOVER Initiative (Researching COVID to Enhance Recovery) funded by NIH. The RECOVER Initiative was created to learn about the long-term effects of COVID. The CRN is proud to be leading three RECOVER trials at Boston Medical Center in partnership with the ReCOVer Long COVID Clinic at BMC, led by Dr. Marathe.
- RECOVER-VITAL: Studying whether Paxlovid can be used to treat Long COVID
- RECOVER-NEURO: Studying possible treatments to improve memory, attention, and brain processing for people who have Long COVID
- RECOVER-SLEEP: studying possible treatments to improve sleep disturbances for people who have Long COVID
We also shared information about the Long COVID Ambassador Training Program, a project led by the MA-CEAL team and supported by the CRN. This initiative utilizes a ‘train-the-trainer’ model to provide education and increase awareness about Long COVID in community-based settings. This is a great opportunity for community health workers, community advocates, engagement teams, and community leaders. Ambassadors will attend a paid training and be prepared to facilitate their own 1-hr workshops within their community, for which they will also be compensated. For more information, please fill out an interest form here: https://redcap.link/LCAT. Upcoming workshops are planned for January 8th and 9th, and February 12th and 13th.
Community members were enthusiastic and eager to learn more about Long COVID research happening at BMC and BUMC. Thank you to all of our amazing partners who made this event possible!
Other Updates:
This is a reminder that the Institutional Standard Operating Procedures (SOPs) for human subjects research are available and effective as of January 1st, 2023. Training will be required to be completed according to the following time-frames:
- January 1, 2024: all new studies targeting BMC patients, utilizing BMC facilities and/or services, or using BMC patient data will require completion of training prior to IRB approval
- January 1, 2024: new BMC employees working on studies within scope are required to complete training within 90-days of employment start date
If there are questions about which level of training to complete, contact the BMC Clinical Research Network (CRN) for guidance. Individual research teams, departments, or other groups wishing to receive customized-SOP training should contact the CRRO for information.
November 2023
Last month, the Clinical Research Network (CRN) attended the 4th Annual Deatrich Wise Block Party at the Josh Kraft Mattapan Teen Center. Despite the rain, it was a great community celebration with fresh food, free haircuts, live music, and many local vendors. BMC partnered with the Boys & Girls Clubs of Boston and New England Patriots' Deatrich Wise for the celebration.
The CRN joined the BMC Community Engagement & External Affairs team, WellSense Health plan, the MA-CEAL team, and the BU CTSI Community Engagement team to provide health & research education, health resources and supplies at the event. We’re proud to join our BMC/BUMC partners in our commitment to serving our communities and providing exceptional care.


October 2023
Upcoming Community Events
Deatrich Wise Block Party
Saturday October 21st | 2:00-6:00pm |Hazelton Street, Mattapan 02126
Boston COVID Recovery Cohort (BCRC) Forum
Policy Priorities to Address Long COVID in Our Country and Advance Health Equity in Our State
Tuesday October 24th | 6:00-8:00pm | Register Here
UCB Partner Night with BU CTSI
Thursday October 26th | 6:00-8:00pm | Zoom
UCB Fall Resource Fair
Saturday November 4th | 10am-12pm | 2300 Washington Street
September 2023

On Saturday August 26, the Clinical Research Network (CRN) attended the Caribbean American Carnival Association of Boston during its 50th Anniversary.
The CRN joined BMC’s Community Engagement & External Affairs, CATALYST, and WellSense Health Plan teams to provide health education and resources to our community. We also provided research education and participant advocacy materials, as well as information on Long COVID, Paxlovid, and APOL1 mediated kidney disease.
The Carnival brought people together in an inclusive space to showcase Caribbean music, art, food, and cultures. There were many activities and events, such as a Caribbean-style J’Ouvert celebration, steelpan competition, masquerade ball, and more!
We're proud to serve our Caribbean families and continue to empower their health through our innovative and equitable programs. They're an integral part of our community as well as Boston's as a whole.
Upcoming Events and those we participated in.
- Saturday September 9th
- Thursday September 14th
- EQTY 2023 BMC Inaugural Summit for Health Justice | Register Here (BMC employees attend free)
- Friday September 15th, 9--10:30 a.m.
- Health, Wealth & Career Success event with Fierce Urgency Now Commerce
- BMC will be hosting a free networking and informational event aimed to equip young professionals, particularly young professionals of color, with the skills, tools, and insights that will advance their careers and financial health while taking care of their mental well-being. Keynote speakers include BMC’s Lovern Moseley, PhD, Michelle Brathwaite from Build Black Wealth, and Leadership Brainery’s Co-Founders Jonathan Allen and Derrick Young Jr. The event will be held on Friday, Sept. 15, from 9-10:30 a.m. at The Record Co. Complementary pastries and coffees will be available. Click here or visit the Hub calendar section to register and learn more about the event.
- Contact: Community Engagement and External Affairs | BMCCommunityEngagement@bmc.org
- Sunday September 17th, 10:00-3:30pm
- Saturday September 23rd, 9:00-1:00pm
- Greater Roslindale Farmers Market with GR Medical & Dental Center
August 2023
The Community Research Network hopes everyone is enjoying their summer! If you’re staying local this month, be sure to check out these upcoming community events – We hope to see you there!
- Family Fun Day at Home for Little Wanderers
- Friday August 11th, 11:00 AM – 4:00 PM
- Community Health Center Week – Kids & Family Day at Greater Roslindale Health Center
- Saturday August 12th , 9:00 AM – 1:30 PM
- Allston-Brighton Open Streets
- Saturday August 19th, 10:00 AM – 3:00 PM
- Madison Park Village Back to School Event
- Wednesday August 23rd, 1:00 PM – 4:00 PM
- Boston Caribbean Carnival at Franklin Park
- Saturday August 26th, 12:00 PM – 3:00 PM
Please email CRN@bmc.org or contact Ryan Schroeder at (ryan.schroeder@bmc.org) with any questions.
July 2023
The CRN team is engaging with our communities to share educational materials and promote awareness of research. All are invited to attend any of these upcoming community events – we’d love to see you out there. Please feel free to share these opportunities within your communities who may benefit from these evets/programs.
If you’re interested in volunteering at an upcoming event, please contact Shannon Timlin, CRN’s Community Engagement & Recruitment Specialist (shannon.timlin@bmc.org). Check back frequently for community engagement activities.
If you are interested in learning more about the CRN’s community engagement and partnerships, please contact us our CRN Clinical Research Coordinator at Olanike.Asupoto@bmc.org or CRN@bmc.org
Upcoming Community Events: (check back frequently for more events.)
- Roxbury Open Streets | Saturday July 15, 10am – 3:30pm
- Blue Hill Avenue, between West Cottage St. to Warren St.
- Roxbury Unity Parade & Block Party | Sunday July 16, 10am – 5pm
- Malcolm X Blvd to Jim Rice Field
- Community Health Week | August 7th – 13th
- More details to come.
Please email CRN@bmc.org or contact Ryan Schroeder at (ryan.schroeder@bmc.org) with any questions.
June 2023
BMC’s Nephrology department and the Clinical Research Network have launched a new trial looking at an investigational drug aimed at treating APOL1-mediated kidney disease (“AMPLITUDE”).
Sponsor: Vertex

PI: Dr. Fola Amodu (Nephrology)
Co-I: Dr. Titi Ilori (Nephrology)
The Clinical Research Network is working with Drs. Amodu and Ilori on a Kidney Disease Study sponsored by Vertex. The study is entitled “Phase 2/3 Adaptive, Double-blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of VX-147 in Subjects Aged 12 Years and Older with APOL1-mediated Proteinuric Kidney Disease.” Individuals with APOL1 mutations are at an increased risk for developing chronic kidney disease, and there are currently no treatments available. This variant of APOL1 is more likely to occur in people of African ancestry. African ancestry includes people who identify as African American, Black, Caribbean, Sub-Saharan African and Latino (Cuban, Mexican, Puerto Rican or South or Central American). VX-147 is a medication designed to decrease proteinuria and evaluate the efficacy on renal function in people diagnosed with kidney disease.
The CRN has consented one patient and is actively screening potential participants. We are also looking forward to engaging with our communities through education and outreach. The CRN is developing a community engagement plan advised by a patient focus group to increase knowledge and awareness about kidney health and kidney disease risk factors, including APOL1-mediated Kidney Disease.
If you are interested in learning more, please contact:
Nike Asupoto; Clinical Research Coordinator II
617-414-7042
olanike.asupoto@bmc.org
Upcoming Community Events:
- G’s 2 Gents: Helping Men Take Steps Toward Self-Empowerment and Personal Growth
- June 10 | 11:00 – 4:00 pm | The Guild – 260 Washington Street 02121
- Juneteenth Block Party with King Boston
- June 16 | 2:00 – 7:00 pm | Roxbury Community College
- Men’s Health Summit 2023: UNITED for Men’s Health: Grounded in Equity
- June 24 | 10:00 – 2:00 pm | Whittier Street Health Center
May 2023
The Clinical Research Network had the honor of presenting at the Society of Research Administrators International iSeries in April to share how BMC is reimagining clinical trial feasibility and rapid study start-up in a community engaged model.
Ryan Schroeder, Director of the Clinical Research Network
Title: Accelerating Study Start-Up – Is it possible to be fast and inclusive?
Description: Time to activate clinical trials has been a primary measure of site performance for decades. In a post COVID-19 pandemic environment where we’ve learned so much about the benefits of rapid activation in a time of emergency, and the inequities caused by research lacking community-engaged strategies to improve diverse recruitment and retention, this webinar will talk about how the largest safety net hospital in New England is attempting to balance both speed and inclusion.
Learning Objectives:
- Learn strategies for reducing study activation timelines.
- Learn how to partner with sponsors to support community-engaged values within a rapid activation model.
Content Level: Intermediate
Duncan Schulte, CRN Regulatory Project Manager
Quinneil Simmons, CRN Program Manager
Title: Feasibility within a Community Engaged Model
Description: Implementing strategic and well-managed feasibility assessments can optimize study goals and participant safety because prioritizing specific metrics in study planning phases is an effort to create a more objective projection of whether or not a study will be successful. However, concurrent with continual advancements in the efficiency of feasibility assessments, is the persistence of a lack of racial, ethnic, and linguistic diversity in clinical trial participation. This is an example of an equity gap that reveals a shortcoming of traditional feasibility analyses in their purpose to project study success. A model of feasibility analysis that has inclusive values and sustainably promotes health equity in the research process is necessary to create the most objective account of the areas where a study can and will be successful. In this model community engagement and community centeredness are ethical imperatives.
Learning objectives:
- Understand a typical feasibility analysis and the value inherent in being engaged during study planning phases.
- Understand a community engaged model of feasibility analysis and its capacity to sustainably promote health equity.
Content level: Intermediate
Please email CRN@bmc.org or contact Ryan Schroeder at ryan.schroeder@bmc.org with any questions.
March 2023
Olanike Asupoto, CRN
Clinical Research Coordinator II
Clinical Research Coordination: Improve diverse participation in research through inclusive engagement through management of screening, consenting and interpreter services coordination.

Duncan Schulte, CRN
Regulatory Project Manager

Quinneil Simmons, CRN
Program Manager

Shannon Timlin, CRN
Community Engagement & Recruitment Specialist

Ryan Schroeder, CRN
Director

Dr. Ben Linas
Medical Director

Meet the CRN Team
After a two-year pilot, the Clinical Research Network has proven vital to BMC’s commitment to inclusive research and providing critical staffing for high-priority trials and under-resourced Investigators.
The Clinical Research Network’s (CRN) mission is to drive and share world-class scientific discovery and innovation through the conduct of leading-edge clinical research that is responsive to cultural and linguistic differences and inclusive of all people.
Clinical Research Network Scope & Services
Community Engagement
- Outreach & Education: Improve community awareness and education to promote diversity in clinical trials and ensure therapies are safe and effective for all people.
- Foster Community-Guided Research: Promote dialogue between our community and researchers so that research is community-guided and focused on health issues most important to those we serve.
- Community Advisory Boards and Focus Groups: Work with internal BMC/BU and community partners to establish advisory boards and patient focus groups that will play an integral role in shaping research at BMC.
Research Oversight and Staffing
CRN provides the staffing resources necessary to rapidly activate and manage our most complex and clinically important studies. The following services are also provided to our researchers, sponsors and the communities BMC serves.
- Regulatory Management: Lead study start up and comprehensive regulatory management.
- Financial Management: Oversee budgeting, invoicing, and expense management.
- Clinical Research Coordination: Manage recruitment, pre-screening, consenting, language/interpreter coordination, data submission.
- Recruitment and Engagement Planning: Guide and deploy study-specific diversity, equity, and inclusion recruitment strategies.
Contact
Contact the Clinical Research Network, Ryan Schroeder, Director at ryan.schroeder@bmc.org.
To join our newsletter mailing list email BMCResearch.Operations@bmc.org.
About Us
The Clinical Research Network’s (CRN) mission is to drive and share world-class scientific discovery and innovation through the conduct of leading-edge clinical research that is responsive to cultural and linguistic differences and inclusive of all people.


