CDW-R Services Hourly Fee Tiers: April 1, 2025
Effective April 1, 2025, all new requests for BMC CDW-R services will be charged at the following hourly fee tiers:
CDW-R will honor the prior fee tier for the existing scope of work on active projects and finalized data requests submitted before April 1, 2025. Continuation, extension of awards, and/or substantial changes in scope will require a new CDW-R data request quoted under the updated fee tiers.
CDW-R remains committed to supporting your research goals. We appreciate your understanding of this necessary update to continue providing high-quality CDW-R services.
Please contact CDW-R at cdw@bmc.org with any questions.
Access Clinical Data for Research
The Boston Medical Center (BMC) Clinical Data Warehouse for Research (CDW-R) is a centralized resource to access patient-level and population-level data for research. CDW-R analysts extract clinical information from BMC's Electronic Health Records (EHR) and other health system-related data streams to leverage data for research. The CDW-R team also collaborates with Departments and Divisions to increase research infrastructure and better leverage data for research purposes.
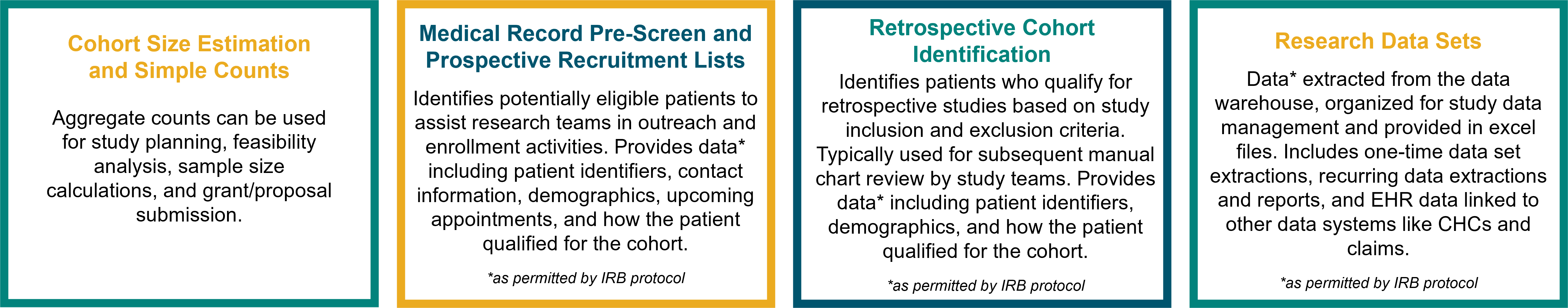
CDW-R Services
Current Wait Times
If you are preparing for a study: CDW-R provides investigators with cohort size simple counts (i.e. aggregated data, not supplied at the patient level) to prepare for a research study or IRB protocol submission. Simple count services are free of charge. CDW-R aims to deliver aggregate data within 7 business days of a finalized request.
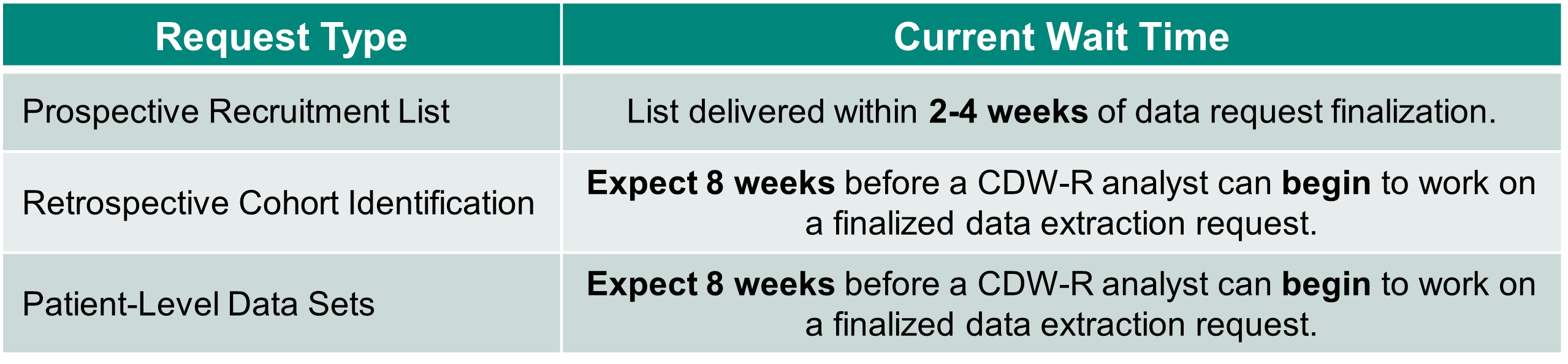
If you need data for a study: Research teams should plan for several weeks between submission of a finalized CDW-R data extraction request (including confirmation of IRB protocol approval/determination, confirmation of any special approvals, a fully completed CDW-R data extraction request form, and identification of an account number to pay for services) and receipt of the data.
Research teams are encouraged to plan according to the wait times below:
Requesting Data
- Read more about Data Available from the CDW-R.
- Read more about CDW-R Data Extraction and Provisioning Services, Data Outputs, and Deliverables.
- Visit How to Request Data for complete the CDW-R research data extraction request process and requirements.
Connect with CDW-R
The CDW-R team is here to help with your research data needs. Whether you need support submitting a research data extraction request or have questions about available data, the CDW-R team is ready to assist you.
- For efficient service, please provide the following when contacting CDW-R: The BMC/BUMC IRB H- Number, a summary of the services/data you anticipate needing from the CDW-R team, and your specific questions.
- For inquiries that cannot be addressed by email, CDW-R offers one (1) initial consultation at no charge for every study. Subsequent consultations and request development support services are billed at our hourly fees.
- All investigators, particularly new investigators or those with early experience working with EHR data, are encouraged to contact CDW-R in advance of IRB submission to confirm available data and discuss feasibility, scope, cost estimates, and how to optimize the data request.
