Supporting and Protecting Clinical Research
As one of two Center for Clinical Research Advancement teams, the Clinical Trial Office (CTO) serves clinical research (CR)* investigators, study teams, and administrators, while also assisting clinical service programs in specialized use of CR systems. We advance BMC’s mission by providing leadership and expertise in the storied complexities of CR billing and finance, thereby protecting our patients in the delivery of exceptional care without exception and upholding our fiduciary responsibilities.
In providing CR financial oversight, the CTO fulfills four purposes. We:
- Act as BMC’s fiscal agent for and signatory official of industry-sponsored agreements;
- Collaborate with Sponsored Programs Administration and Sponsored Programs Finance on relevant awards;
- Direct internally and externally funded investigator-initiated research; and
- As a shared service, guide BUMC’s investigators and study teams.
Among our compliance-assurance operational duties are:
- Reviewing, negotiating, and approving all CR agreements;
- Ensuring compliant protocol billing planning and execution;
- Maintaining research pricing of clinical services;
- Monitoring account finances; and
- Providing expertise on CR billing and finance management systems.
Oversee CR financials | Ensure compliant billing | Support our university colleagues
* For the purpose of identifying the range of CTO’s financial oversight, “clinical research” is defined as any study whose IRB protocol includes one or more prospective clinical services, the provision of which is subject to federal law, regulation, and coverage determinations.
Clinical Trials Office
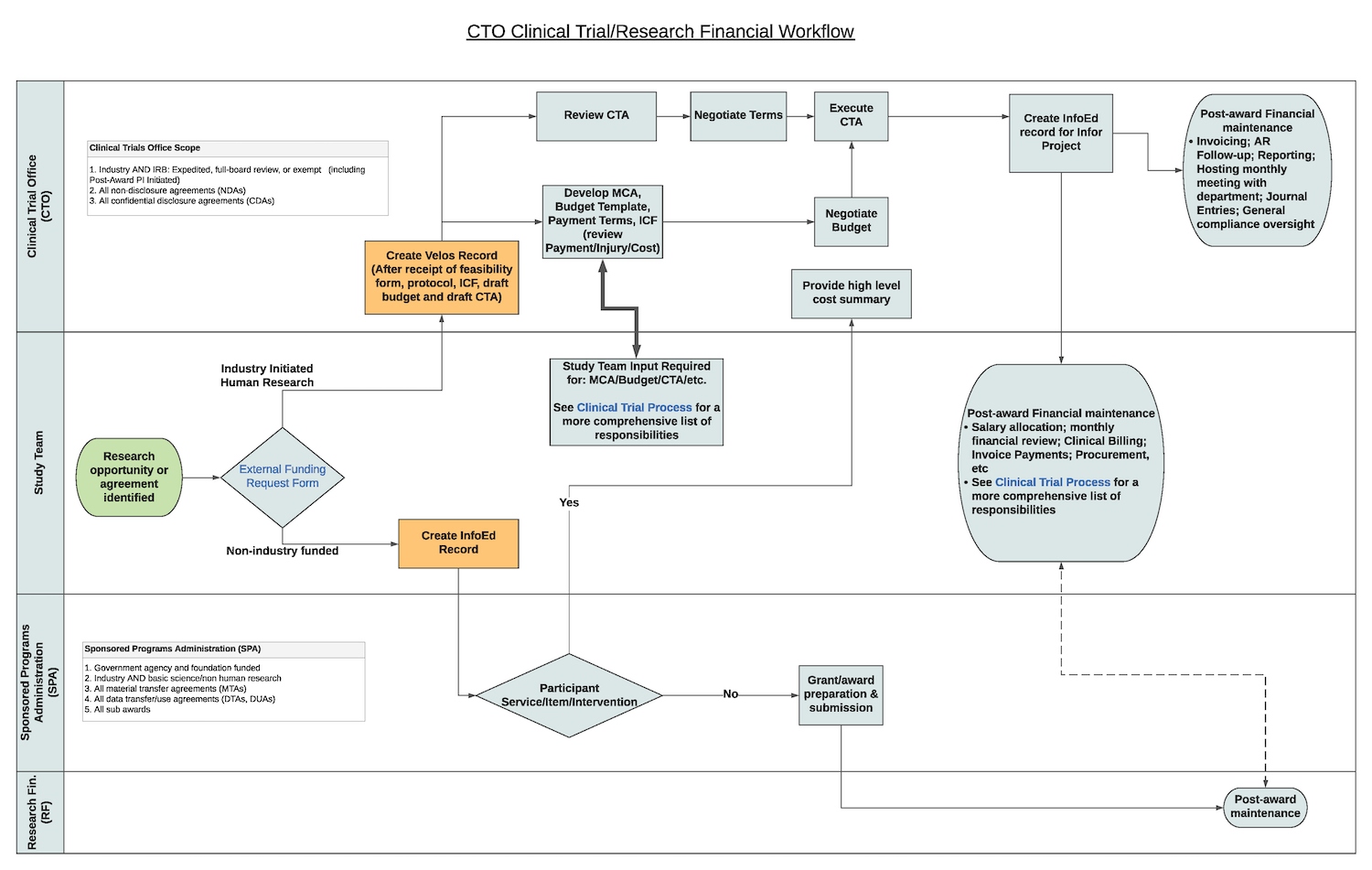
CTO Clinical Trial Research Financial Workflow
Frequently Asked Questions
How do I initiate a clinical trial agreement (CTA)?
The first step is to complete an CTO Intake Form, which allows the Clinical Trial Office (CTO) to complete an initial assessment of the study proposed, including study events that need to be captured in VelosCT, the clinical trial management system (CTMS) of BMC and Boston University Medical Campus (BUMC).
As needed, the CTO will request that you set up your study in VelosCT for management of the contract negotiation. Once the draft CTA, budget, and informed consent form (ICF) are uploaded, and the study summary and other details are completed, then your assigned Financial Analyst should reach out to you about next steps on our initiates initial review, coverage analysis, and contract negotiation.
Why do I need to complete the External Funding Request Form?
The External Funding Request Form provides details needed for initial assessment of your proposed study, allowing us to discern the necessary steps toward launching your project efficiently, while meeting all governmental and institutional requirements.
What is a coverage analysis?
Coverage analysis (CA) is a crucial part of clinical research billing (CRB) compliance, which is necessary to participant protections. Often formerly called "Medicare" coverage analysis (MCA), because the Centers for Medicare and Medicaid Services (CMS) was the first insurer to explicitly allow clinical trial billing of routine services, CA reviews protocol-specified clinical services -- identified as such or included under study administration/effort-- for whether they are billable to insurance or must be billed to the study. It further determines the necessary claims modifications that Medicare instituted for data collection and audit purposes, which many insurers have since adopted. CA is necessary to participant protection and compliant billing, but it also a negotiating tool to align sponsor interests with CRB compliance.
Why do I need to use VelosCT for my clinical trial?
To ensure clinical research billing (CRB) compliance of BMC and BU studies, the CTO employs VelosCT, a clinical trial management system (CTMS), which is integrated with Epic, BMC's electronic medical record (EMR). Because CRB operates at the visit and participant level, VelosCT saves time and risk. In addition, VelosCT allows for study metrics that are used for resource development and sponsor collaboration.
What is the process of getting a clinical trial agreement (CTA) executed?
After a protocol is shared through the confidentiality disclosure/non-disclosure agreement (CDA/NDA), and the study is confirmed as desirable and practical, CTO staff initiates the CTA process, including coverage analysis (CA), budget negotiation, negotiation of CTA terms and conditions, and harmonization of the informed consent language. Once document harmonization is achieved, and the sponsor and BMC concur, the CTA is signed and is thus "fully executed."